| AMMONIUM FLUORIDE | |||
|
PRODUCT IDENTIFICATION |
|||
| CAS NO. | 12125-01-8 |
|
|
| EINECS NO. | 235-185-9 | ||
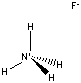
| FORMULA | NH4F | ||
| MOL WT. | 37.04 | ||
|
H.S.CODE |
|||
| TOXICITY | |||
| SYNONYMS | (NH4)F; Ammonium fluorure; Fluorure d'ammonium; Fluoruro amonico; | ||
| Neutral ammonium fluoride; Other CAS RN.: 15818-75-4; 16099-75-5; 70786-97-9; 102089-31-6; 124633-37-0; 133332-88-4; 153985-02-5; 161288-32-0; 178115-94-1; 204322-87-2; 215303-13-2; 357290-87-0; 502760-32-9; 950196-00-6; | |||
| SMILES | |||
|
CLASSIFICATION |
|
||
|
PHYSICAL AND CHEMICAL PROPERTIES |
|||
| PHYSICAL STATE | white crystals | ||
| MELTING POINT | Decomposes | ||
| BOILING POINT | |||
| SPECIFIC GRAVITY | |||
| SOLUBILITY IN WATER | 100g/l00 ml | ||
| pH | aqueous solution is weak acidic | ||
|
AUTOIGNITION |
|
||
| NFPA RATINGS | |||
|
REFRACTIVE INDEX |
|
||
| FLASH POINT | Non-flammable | ||
| STABILITY | Stable under ordinary conditions of use and storage | ||
|
GENERAL DESCRIPTION & APPLICATIONS |
|||
Ammonium fluoride is a white crystalline solid soluble in water, slightly soluble in alcohol. Ammonium fluoride adopts the wurtzite crystal structure, in which both the ammonium cations and the fluoride anions are stacked in ABABAB... layers, each being tetrahedrally surrounded by four of the other. It is noncombustible but decomposes to ammonia and hydrogen fluoride when heated and to ammonia and ammonium bifluoride in hot water. It reacts with chlorine trifluoride causing explosion hazard and attacks glass and metal. It is corrosive to aluminum. This substance can cause fluoride poisoning. This compound is commonly called 'neutral ammonium fluoride' to represent the neutral salt - [NH4]F. As the acid salt contains a higher percentage of fluoride by mass, it is usually used in preference to the neutral salt in the etching of glass. Ammonium fluoride is used in:
- Glass
etching |
|||
| SALES SPECIFICATION | |||
|
APPEARANCE |
White needle crystal | ||
| NH4F | 98.0% min | ||
| SO4 | 0.1% max | ||
| SiF6 |
2.0% max |
||
| RESIDUE ON IGNITION | 0.15% max | ||
| TRANSPORTATION | |||
| PACKING | 25kgs per bag | ||
| HAZARD CLASS | 6.1 | ||
| UN NO. | 2505 | ||
| OTHER INFORMATION | |||
| Hazard Codes: T, Risk Statements: 23/24/25, Safety Statements: 26-45 | |||
|
PRICES |
|||
|
|
|||